Dye Sensitized Solar Cell with Immersion Time Variation of Working Electrode on Quantum Dot: A Performance Optimization Study
DOI:
https://doi.org/10.70882/josrar.2025.v2i5.116Keywords:
Dye-sensitized Solar Cells, TiO₂, Quantum Dot, EfficiencyAbstract
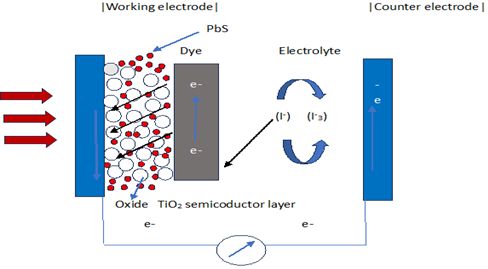
The growing demand for renewable and sustainable energy has spurred interest in dye-sensitized solar cells (DSSCs) as cost-effective alternatives to conventional silicon solar cells. However, their performance is often limited by narrow spectral absorption and dye instability. This study investigates the optimization of DSSC performance by incorporating lead sulfide (PbS) quantum dots (QDs) and natural hibiscus dye as a hybrid photo-sensitizer. QD-DSSC devices were fabricated using TiO₂-coated electrodes sensitized with hibiscus dye and 0.05 M PbS quantum dots at varying immersion times (6 h, 12 h, 24 h). The I–V characterization revealed that immersion time critically influenced device performance. The highest efficiency of 3.19 % was achieved at 12 hours of immersion, corresponding to the optimal balance of dye loading and PbS layer formation. The associated photovoltaic parameters were Isc = 7.965 mA, Voc = 0.72 V, and FF = 0.557, indicating improved electron transport and reduced recombination. In contrast, 6 h and 24 h immersion times yielded lower efficiencies of 2.01 % and 0.754 %, respectively, due to insufficient or excessive sensitizer deposition leading to poor charge mobility or increased recombination. Electrical performance analysis using a solar simulator and electrochemical impedance spectroscopy (EIS) confirmed that 12-hour immersion provided optimal interfacial charge transfer and minimized resistance. The study demonstrates that integrating PbS QDs with natural dyes broadens spectral absorption and enhances DSSC efficiency, with 12 hours identified as the optimal immersion duration for sensitizer application.
References
Alhamed, M., Issa, A. S. and Doubal, A. W. (2012). Studying of natural dyes properties as photo-sensitizer for dye sensitized solar cells (DSSC). Journal of electron Devices, 16(11): 1370-1383.
Andreani, L. C., Bozzola, A., Kowalczewski, P., Liscidini, M. and Redorici, L. (2019). Silicon solar cells: toward the efficiency limits. Advances in physics: X, 4(1): 1548305.
Bisquert, J. (2004). Chemical diffusion coefficient of electrons in nanostructured semiconductor electrodes and dye-sensitized solar cells. The Journal of Physical Chemistry B, 108(7): 2323-2332.
Brus, L. E. (1984). Electron–electron and electron‐hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. The Journal of chemical physics, 80(9): 4403-4409.
Calogero, G., Di Marco, G., Cazzanti, S., Caramori, S., Argazzi, R., Di Carlo, A. and Bignozzi, C. A. (2010). Efficient dye-sensitized solar cells using red turnip and purple wild Sicilian prickly pear fruits. International journal of molecular sciences, 11(1): 254-267.
Du, L., Furube, A., Yamamoto, K., Hara, K., Katoh, R. and Tachiya, M. (2009). Plasmon- induced charge separation and recombination dynamics in gold− TiO2 nanoparticle systems: dependence on TiO2 particle size. The Journal of Physical Chemistry C, 113(16): 6454-6462.
Gao, J., Hu, X., Zhang, L., Li, F., Zhang, L., Wang, Y. and Ren, X. (2014). Major contributor to the large piezoelectric response in (1− x) Ba (Zr0. 2Ti0. 8) O3− x (Ba0. 7Ca0. 3) TiO3 ceramics: domain wall motion. Applied Physics Letters, 104:25
Giusti, M. M. and Wrolstad, R. E. (2001). Characterization and measurement of anthocyanins by UV‐visible spectroscopy. Current protocols in food analytical chemistry, 1 :1-2.
Gong, J., Liang, J. and Sumathy, K. (2012). Review on dye-sensitized solar cells (DSSCs): Fundamental concepts and novel materials. Renewable and Sustainable Energy Reviews, 16(8): 5848-5860.
Grätzel, M. (2001). Photoelectrochemical cells. nature, 414(6861): 338-344.
Grätzel, M. (2005). Solar energy conversion by dye-sensitized photovoltaic cells. Inorganic chemistry, 44(20): 6841-6851.
Hines, M. A. and Guyot-Sionnest, P. (1996). Synthesis and characterization of strongly luminescing ZnS-capped CdSe nanocrystals. The Journal of Physical Chemistry, 100(2): 468-471
Hodes, G. (2007). When small is different: some recent advances in concepts and applications of nanoscale phenomena. Advanced Materials, 19(5): 639-655.
Holick, M. F. (2016). Biological effects of sunlight, ultraviolet radiation, visible light, infrared radiation and vitamin D for health. Anticancer research, 36(3): 1345-1356.
Hosenuzzaman, M., Rahim, N. A., Selvaraj, J., Hasanuzzaman, M., Malek, A. A. and Nahar, A. (2015). Global prospects, progress, policies, and environmental impact of solar photovoltaic power generation. Renewable and sustainable energy reviews, 41: 284-297.
Kamat, P. V. (2007). Meeting the clean energy demand: nanostructure architectures for solar energy conversion. The Journal of Physical Chemistry C, 111(7): 2834-2860.
Kamat, P. V. (2008). Quantum dot solar cells. Semiconductor nanocrystals as light harvesters. The Journal of Physical Chemistry C, 112(48): 18737-18753.
Keitel, R. C., Weidman, M. C. and Tisdale, W. A. (2016). Near-infrared photoluminescence and thermal stability of PbS nanocrystals at elevated temperatures. The Journal of Physical Chemistry C, 120(36): 20341-20349.
Kim, J. Y., Lee, K. J., Kang, S. H., Shin, J.m and Sung, Y. E. (2011). Enhanced photovoltaic properties of a cobalt bipyridyl redox electrolyte in dye-sensitized solar cells employing vertically aligned TiO2 nanotube electrodes. The Journal of Physical Chemistry C, 115(40): 19979-19985.
Kim, J., Koh, J. K., Kim, B., Kim, J. H. and Kim, E. (2012). Nanopatterning of Mesoporous Inorganic Oxide Films for Efficient Light Harvesting of Dye‐Sensitized Solar Cells. Angewandte Chemie International Edition, 51(28): 6864-6869.
Kim, T., Kim, J. H., Kang, T. E., Lee, C., Kang, H., Shin, M. and Kim, B. J. (2015). Flexible, highly efficient all-polymer solar cells. Nature communications, 6(1): 8547.
Koleilat, G. I., Levina, L., Shukla, H., Myrskog, S. H., Hinds, S., Pattantyus-Abraham, A. G. and Sargent, E. H. (2008). Efficient, stable infrared photovoltaics based on solution-cast colloidal quantum dots. ACS nano, 2(5): 833-840.
Konstantatos, G. and Sargent, E. H. (2010). Nanostructured materials for photon detection. Nature nanotechnology, 5(6): 391-400.
Lai, W. H., Su, Y. H., Teoh, L. G. and Hon, M. H. (2008). Commercial and natural dyes as photosensitizers for a water-based dye-sensitized solar cell loaded with gold nanoparticles. Journal of Photochemistry and Photobiology A: Chemistry, 195(2-3):307- 313.
Law, M., Greene, L. E., Johnson, J. C., Saykally, R. and Yang, P. (2005). Nanowire dye-sensitized solar cells. Nature materials, 4(6): 455-459
Lee, H. J., Liu, Y., Coull, B. A., Schwartz, J. and Koutrakis, P. (2011). A novel calibration approach of MODIS AOD data to predict PM 2.5 concentrations. Atmospheric Chemistry and Physics, 11(15):7991-8002.
Li, G., Zhu, R. and Yang, Y. (2012). Polymer solar cells. Nature photonics, 6(3):153-161.
Liu, C. Y., Holman, Z. C. and Kortshagen, U. R. (2009). Hybrid solar cells from P3HT and silicon nanocrystals. Nano letters, 9(1): 449-452.
Liu, J., Wang, J., Liu, Y., Xian, K., Zhou, K., Wu, J. and Ye, L. (2023). Toward efficient hybrid solar cells comprising quantum dots and organic materials: progress, strategies, and perspectives. Journal of Materials Chemistry A, 11(3):1013-1038.
Luther, J. M., Law, M., Beard, M. C., Song, Q., Reese, M. O., Ellingson, R. J. and Nozik, A. J. (2008). Schottky solar cells based on colloidal nanocrystal films. Nano letters, 8(10): 3488-3492.
Nozik, A. J. (2002). Quantum dot solar cells. Physica E: Low-dimensional Systems and Nanostructures, 14(1-2): 115-120.
O'regan, B. and Grätzel, M. (1991). A low-cost, high-efficiency solar cell based on dye- sensitized colloidal TiO2 films. nature, 353(6346):737-740.
Sargent, E. (2005). Infrared quantum dots. Advanced Materials, 17(5): 515-522.
Shah, A. V., Platz, R. and Keppner, H. (1995). Thin-film silicon solar cells: a review and selected trends. Solar energy materials and solar cells, 38(1-4):501-520.
Sharma, S., Jain, K. K. and Sharma, A. (2015). Solar cells: in research and applications—a review. Materials Sciences and Applications, 6(12):1145-1155.
Tang, H., Liang, D., Qiu, R. L. and Gao, X. P. (2011). Two-dimensional transport-induced linear magneto-resistance in topological insulator Bi2Se3 nanoribbons. ACS nano, 5(9): 7510-7516.
Trihutomo, P., Marji, M., Harly, M., Wahyudi, B. A. and Radja, M. B. (2022). The effect of Clathrin protein addition on increasing the number of electrons in organic Dye-Sensitized Solar Cell (DSSC). EUREKA: Physics and Engineering, 2:15-27.
Wang, X., Weng, Q., Yang, Y., Bando, Y. and Golberg, D. (2010). Hybrid two-dimensional materials in rechargeable battery applications and their microscopic mechanisms. Chemical Society Reviews, 45(15): 4042-4073.
Wong, J. H., Royapoor, M. and Chan, C. W. (2016). Review of life cycle analyses and embodied energy requirements of single-crystalline and multi-crystalline silicon photovoltaic systems. Renewable and sustainable energy reviews, 58:608-618.
Yella, A., Lee, H. W., Tsao, H. N., Yi, C., Chandiran, A. K., Nazeeruddin, M. K. and Grätzel, M. (2011). Porphyrin-sensitized solar cells with cobalt (II/III)–based redox electrolyte exceed 12 percent efficiency. science, 334(6056): 629-634.
Yu, W. W., Qu, L., Guo, W. and Peng, X. (2003). Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chemistry of materials, 15(14): 2854-2860.
Zhang, C., Huang, Y., Huo, Z., Chen, S. and Dai, S. (2009). Photoelectrochemical effects of guanidinium thiocyanate on dye-sensitized solar cell performance and stability. The Journal of Physical Chemistry C, 113(52): 21779-21783.
Zhang, Q., Jin, T., Ye, X., Geng, D., Chen, W. and Hu, W. (2021). Organic field effect transistor‐based photonic synapses: materials, devices, and applications. Advanced Functional Materials, 31(49):2106151.
Zhang, Y., Kan, Y., Gao, K., Gu, M., Shi, Y., Zhang, X. and Jen, A. K. Y. (2020). Hybrid quantum dot/organic heterojunction: A route to improve open-circuit voltage in PbS colloidal quantum dot solar cells. ACS Energy Letters, 5(7): 2335-2342.
Zhao, D. W., Liu, P., Sun, X. W., Tan, S. T., Ke, L. and Kyaw, A. K. K. (2009). An inverted organic solar cell with an ultrathin Ca electron-transporting layer and MoO3 hole-transporting layer. Applied Physics Letters, 95: 15
Zhou, R., Niu, H., Ji, F., Wan, L., Mao, X., Guo, H. and Cao, G. (2016). Band-structure tailoring and surface passivation for highly efficient near-infrared responsive PbS quantum dot photovoltaics. Journal of Power Sources, 333: 107-117.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Journal of Science Research and Reviews

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
- Attribution — You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
- NonCommercial — You may not use the material for commercial purposes.
- No additional restrictions — You may not apply legal terms or technological measures that legally restrict others from doing anything the license permits.




